Summary
Increased protein oxidation, nitration and glycation of cartilage have long been implicated as damaging modifications in the joint mediating development of arthritic disease. There were few studies on protein damage in early-stage disease. Experimental studies of gene deletion and pharmacological activation of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor which coordinates basal and inducible expression of enzymes protective against oxidative stress and dicarbonyl glycation, supports a role of oxidative damage and dicarbonyl glycation in development of osteoarthritis (OA) and rheumatoid arthritis (RA). Trace level oxidation, nitration and glycation free adducts potentially provide a report of this in plasma. This is particularly important for detection of early-stage disease. Acting on detection of early-stage RA (eRA), use of disease-modifying anti-rheumatic drugs (DMARD) has produced the prospect of a therapeutic cure of RA. Severe life impairment by development of OA may likely be prevented if arthritic disease is identified and treated in the early stages. Currently, magnetic resonance imaging techniques have been developed for evaluation of cartilage damage in early-stage OA (eOA). These imaging techniques have approximately 70% sensitivity and 90% specificity compared to reference diagnosis by arthroscopy. They require expensive instrumentation time and facilities. They cannot be used in patient populations with implanted pacemakers or aneurysm coils. Experience in the detection of RA by clinical chemistry assessments has improved by introduction of the anti-cyclic citrullinated peptide (CCP) antibody test which has sensitivity 68% and specificity 98% for established disease. The presumed antigen is citrullinated protein (CP) which we found increased in both eRA and eOA with only dominant immunogenicity in the former. The anti-CCP antibody test is now considered state-of-the-art in RA diagnosis. It has relatively low sensitivity for eRA of 61% and hence improvement is desirable.
In searching for biomarkers for clinical diagnosis, analysis of proteins is often considered and preferred over amino acids since proteins are typically longer-lived than amino acids and may be linked functionally to mechanism of the target disease. Restricted access to samples of affected tissues often precludes measurement of target tissue-based proteins. Proteins damaged by oxidation, nitration and glycation undergo proteolysis to release oxidized, nitrated and glycated amino acids – also called oxidation, nitration and glycation free adducts. Increase in such trace level damaged amino acids in synovial fluid equilibrates rapidly with plasma. Amino acids may be detected with high analytical specificity and sensitivity and robustly quantified by stable isotopic dilution analysis liquid chromatography-tandem mass spectrometry (LC-MS/MS). Plasma levels of oxidized, nitrated and glycated amino acids may thereby provide signatures of protein damage, dysfunction and disease progression in the joint in early and advanced stage arthritis. Combination with the biomarker of bone turnover and resorption – hydroxyproline (Hyp) and anti-CCP antibody status, the signature mat be made specific for arthritic disease.
Assay of citrullinated protein in plasma protein and 4-way classification of early-state arthritis
We initially developed a stable isotopic dilution analysis LC-MS/MS assay method for assay of citrullinated protein (CP) in plasma. This revealed that CP was increased in eRA and eOA but not in subject with good skeletal health and patients with inflammatory joint disease which is often self-resolving or non-rheumatoid arthritis (non-RA). Plasma hydroxyproline (hyp) was increased in eRA, eOA and non-RA. Together with anti-cyclic citrullinated peptide (CCP) antibody status – positive in eRA, these measurements allowed for training and testing of a 4-class algorithm for diagnosis and classification of early-stage arthritis.
Summary scheme

Figure 1. Citrullinated protein, plasma hyp and anti-CCP antibody status for diagnosis and classification of early-stage arthritis (a) Formation of citrullinated protein and analysis of citrullinated protein. (b) Detection and quantitation of citrulline by stable isotopic dilution analysis LC-MS/MS. Left-hand panel – chromatogram of citrulline detection in plasma protein digest form patient with eOA, middle panel – chromatogram of [5-13C-4,4,5,5-2H4]citrulline internal standard, right-hand panel – calibration curve of citrulline. (c) CP and (d) Hyp in early-stage arthritis and healthy human subjects. Significance: *, ** and ***, P<0.05, P<0.01 and P<0.001 with respect to control (healthy people). Data are median (lower – upper quartile). (e) Discrimination of study groups by discrete patterns of CP, Hyp and Anti-CCP antibody response. Each darkened shade presents approximately a 2-fold increase.

Figure 2. Citrullinated protein, plasma hyp and anti-CCP antibody status for diagnosis and classification of early-stage arthritis – classifier algorithm Training and validation of a multiclass algorithm for detection and discrimination of early stage osteoarthritis, rheumatoid arthritis, other inflammatory joint disease from healthy subjects. (a) Training set and test set study groups. Receiver operating characteristic curves in the test set for detection of: (b) healthy controls, (c) eOA, (d) eRA and (e) non-RA.
Principal publication
Ahmed, U., Savage, R.S., Anwar, M.M., Costa, M.L., Filer, A., Raza, K., Watts, R.A., Winyard, P.G., Tarr, J., Haigh, R.C., Thornalley, P.J. and Rabbani, N. (2015) Biomarker combination detects early-stage and discriminates osteoarthritis, rheumatoid arthritis and other inflammatory joint disease. Scientific Reports 5: 9259.
Assay of plasma glycated, oxidized and nitrated amino acids for diagnosis and classification of early-stage clinical arthritis
Summary
We hypothesized that damage to proteins of the joint in early-stage arthritis by oxidation, nitration and glycation would release diagnostic signatures of oxidized, nitrated and glycated amino acids into plasma which may facilitate early-stage diagnosis and typing of arthritis. Patients with knee joint early-stage and advanced osteoarthritis (OA) and rheumatoid arthritis (RA), other inflammatory joint disease (non-RA) and healthy subjects with good skeletal health were recruited for the study. Plasma/serum and synovial fluid was analyzed for oxidized, nitrated and glycated proteins and amino acids by quantitative liquid chromatography-tandem mass spectrometry. Data-driven machine learning methods were employed to explore diagnostic utility of the measurements for detection and classifying early-stage OA and RA, non-RA and good skeletal health with training set and independent test set cohorts.
Glycated, oxidized and nitrated proteins and amino acids were detected in synovial fluid and plasma of arthritic patients with characteristic patterns found in early and advanced OA and RA, and non-RA, with respect to healthy controls. In early-stage disease, two algorithms for consecutive use in diagnosis were developed: (i) disease-versus-healthy control, and (ii) classification as OA, RA and non-RA. The algorithms featured 10 plasma damaged amino acids, hydroxyproline and anti-CCP antibody status. Sensitivities/specificities were: (i) 0.92/0.91 good skeletal health; (ii) early-stage OA, 0.92/0.90; early-stage RA, 0.80/0.78; and non-RA, 0.70/0.65 (training set). These were confirmed in independent test set validation. Damaged amino acids increased further in severe and advanced OA and RA. Oxidized, nitrated and glycated amino acids combined with hydroxyproline and anti-CCP antibody status provided a plasma-based biochemical test of relatively high sensitivity and specificity for early-stage diagnosis and typing of arthritic disease.
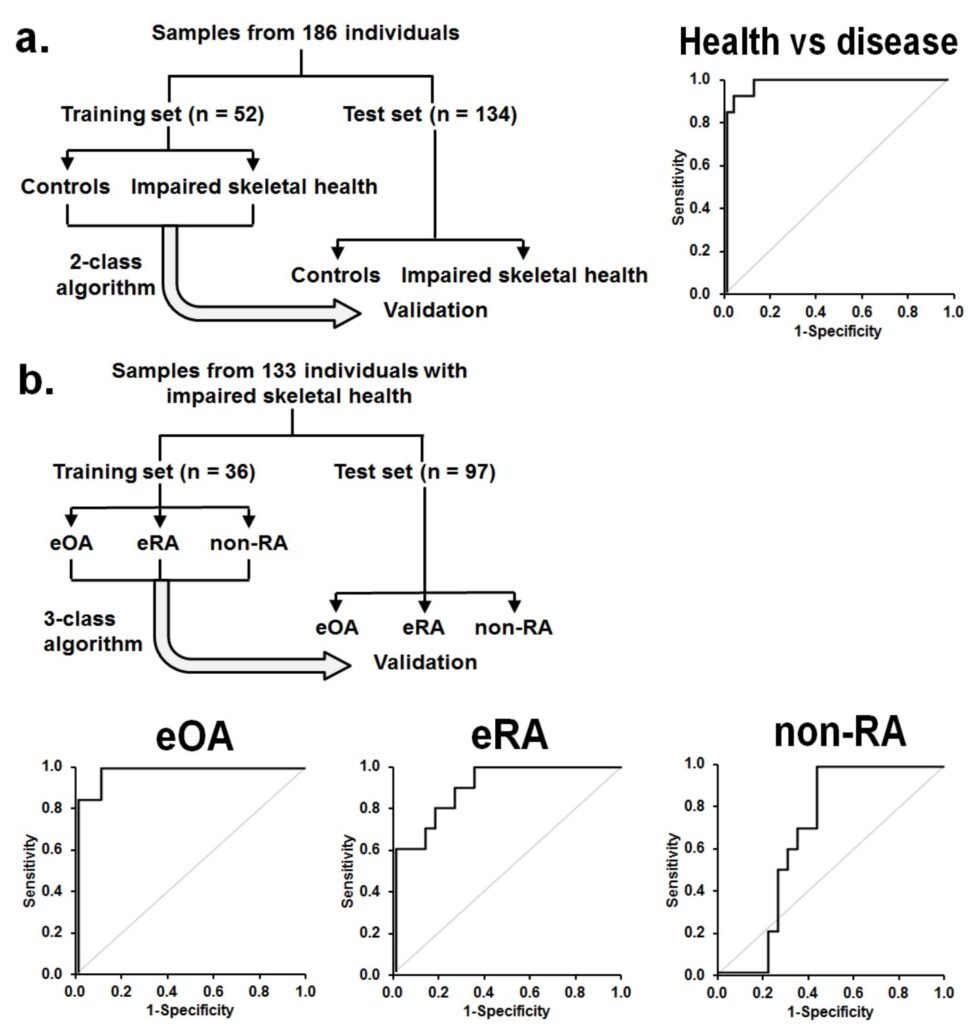
Figure 1. Assay of plasma glycated, oxidized and nitrated amino acids for diagnosis and classification of early-state clinical arthritis. Training and validation of 2-stage diagnostic algorithms for detection of impaired skeletal health and discrimination of eOA, eRA and non-RA.
a Training set and test set study groups for detection of impaired skeletal health. ROC curve is given for the training set. AUROC was: 0.99. Comparators: early-stage arthritis (eOA + eRA + nonRA) versus healthy controls. A random outcome is 0.50.
b Training set and test set study groups for discrimination of eOA, eRA and non-RA.ROC curves are given for the training set with AUROC and confidence intervals: eOA, 0.98; eRA, 0.91; and non-RA, 0.68. A random outcome is 0.33.
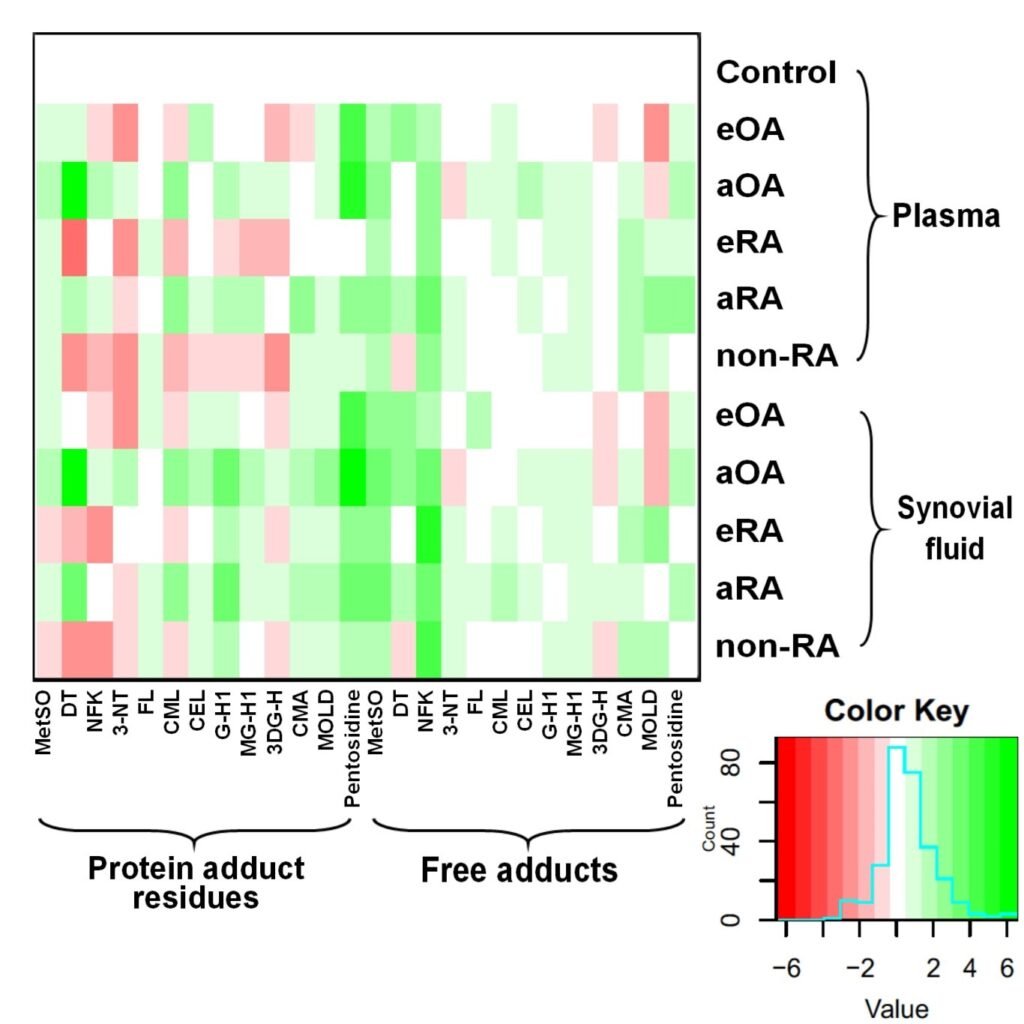
Figure 2. Assay of plasma glycated, oxidized and nitrated amino acids for diagnosis and classification of early-state clinical arthritis. Heat map of changes in glycated, oxidized and nitrated proteins and amino acids in plasma and synovial fluid of patients with early and advanced arthritis.
Principal publications
Ahmed, U., Anwar, A., Savage, R.S., Thornalley, P.J. and Rabbani, N. (2016) Application of protein glycation, oxidation and nitration for early-stage diagnosis and severity of osteoarthritis and other arthritic disease. Arthritis Research & Therapy 18:250 Catherine Legrand, Usman Ahmed, Attia Anwar, Kashif Rajpoot, Sabah Pasha, Cécile Lambert, Rose K. Davidson, Ian M. Clark, Paul J. Thornalley, Yves Henrotin and Naila Rabbani (2018) Glycation marker glucosepane increases with the progression of osteoarthritis and correlates with morphological and functional changes of cartilage in vivo Arthritis Research & Therapy 20.
